Material and Method
Sampling occurred on Bökestorp farm, Skåne County. Ethical permits for the experimental procedures were approved by the ethical committee of Malmö-Lund (Dnr. M 71-14).
Two groups sampled at 9 (N=121) and 12 weeks of age (N=120):
- SKIP (5:2 skip-a-day)
- CTRL (every-day)
Body mass, tonic immobility (TI), novel object (NO), plasma glucose, activity and blood smears for H/L ratios was sampled for each individual and liver mass and liver glycogen was sampled from a total on 40 individuals
Body mass
All sampled chickens were weighed upside-down, hanging by their legs using a Weltech weighing system (BW-2050, Weltech International Ltd, St. Ives, Cambridgeshire, UK), with a shackle.
Novel object (NO)
A novel object-test was carried out according to the description provided in the Welfare Quality (2009) assessment protocol for poultry.
A 40 cm wooden rod, taped with 5 different colours of 2 cm wide insulating tape, was dropped in one area of a pen after standing immobile in that area for 5 min of acclimatisation.
When the rod had been dropped, the test persons backed approximately 2 m and started to record the number of chickens closer than a bird length to the NO every 10 s for a total of 2 min.

Tonic immobility (TI)
One hen at a time was tested by the same test person. For inducing the TI, the hen was turned on its back in a V-shaped wood construction with one hand covering the head and with the other hand gently pressing the sternum. After 15 s, the test person removed the hands and backed approximately 1 m, and started to record time after 5 s of immobility. The time for first head movement was noted as well as the time until self-righting (TI duration).

Activity loggers
Six activity loggers (Motion Watch 8, CamNtech, Cambridge, UK) were applied to the CTRL- and the SKIP group with 3 loggers on 3 chickens in each group. The activity loggers were worn as backpacks that were made out of cohesive bandage and rubber bands threaded over the wing and applied at the wing base.

Blood sampling
Blood was collected by puncturing the ulnar vein underneath the left wing with a syringe and was then obtained using a 200 µl EDTA prepared blood collection tube with a capillary cap.
Glucose
One droplet of blood from the capillary cap of the EDTA collection tube was analysed for glucose using an Accu-Chek Aviva Plus glucometer (Roche Diabetes Care, Inc., Indianapolis, Indiana, USA) with matching test strips.
Preparing a blood smear
Pipette 2 µl blood on a glass slide and use another glass slide as a spreader. The spreader is put in front of the droplet and then backed up into the droplet. When the blood is distributed along the edge, the spreader is held at approximately 45-degree angle and then drawn forwards with a constant pace to ensure an even distribution of blood cells.
Staining a blood smear
Fixate smears in 100 % methanol and stain in a laboratory within 7 days of preparation. Stain smears in May-Grünwald-Giemsa solution.
Dilute the May-Grünwald solution 1:2 with tap water and stain smears in this solution for 5 min.
Dilute the Giemsa solution 1:10 with tap water and stain smears for 30 min in this solution directly after staining in May-Grünwald.
Rinse all smears in tap water for 30-60 s after staining and then leave them to air dry.
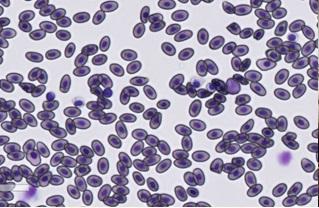
Counting H/L ratios
Smears were scanned using a digital scanner (NanoZoomer 2.0-HT Digital slide scanner C9600, Hamamatsu Photonics, Hamamatsu, Japan), and examined using Aperio ImageScope© (version 12.2.2.5015, Leica biosystems, Nussloch, Eisfeld, Germany).
Leukocytes to be counted included heterophils, lymphocytes, monocytes, eosinophils and basophils.
In this project, approximately 100 leukocytes were counted within a preselected and marked area in 20-40 x magnification of the scan. The ratio of heterophils and lymphocytes was determined by dividing the counted number of heterophils with the counted number of lymphocytes.
Liver mass and liver glycogen
- 40 breeder hens were sacrificed at 12 weeks of age
- 28 individuals from the SKIP group
- 12 individuals from the CTRL group
The birds were sacrificed by cutting the carotid artery and crushing the spinal cord with hedge shears and then let to be bled. All sacrificed individuals were sampled for liver mass and the liver were then stored in a cryoshipper at -80°C.
- The livers were thawed in a laboratory and 300 mg were homogenized in perchloric acid (HClO4) in order to prevent glycogen breakdown.
- The supernatant was separated and washed in petroleum ether and then kept at -80°C until further analysis.
- The sample was defrosted, centrifuged and decanted. A colour solution (10.4 mg I 2 , 104 mg KI and 3 g CaCl 2 in 30.4 ml deionized water) was then added to the extract.
- The absorbance was measured at 450 nm after 10-30 min of colour solution and then compared to a standard curve consisting of bovine liver glycogen (G0885-25G, Type IX, Sigma-Aldrich Sweden AB, Stockholm, Sweden).
Responsible for this page:
Director of undergraduate studies Biology
Last updated:
05/17/16
