Background
The nervous system is extremely complex which contains myriad variety of cell subtypes. Human nerve system is the most complicated one with approximately 10 11 cells divided in to 10,000 subtypes and they differ from one another in many aspects like their morphology, type of neurotransmitters they release as well as their expression pattern of receptors and ion channels. Noticeably all of these different cell types take origin from stem cells (neuroblasts) trough multiple regulatory steps, mostly occur during early developmental stages.
The central nervous system of D. melanogaster is quite complex and it has comparable basic aspects of neuronal development with humans and a number of basic genetic elements are shared between both of them, although their common ancestor had been a lived about 550 million years ago (Erwin & Davidson 2002).
These basic common elements take charge of highly evolutionary conserved mechanisms like, cell cycle regulation, adhesion and signaling pathways also apoptosis and innate immunity (Rubinet et al. 2000).
Additionally, the D. melanogaster genome contains of more than 84% euchromatic genes (Kaufman 2010). Moreover, 75% of human genes involved in different diseases have fruit fly orthologs genes (Reiter et al. 2001). Among all, Wnt, Hedgehog and Notch are two pathways in common between fruit fly and humans with key role in embryonic development.
Hence D. melanogaster was opted as the model for this thesis, However, the D. melanogaster CNS is still too complex to be studied wholely; to come up with a solution, a small and well-studied part of fruit fly embryonic CNS (Apterous cluster) is concerned as the model within the model in this project.
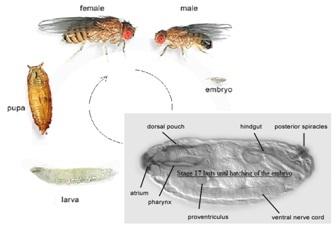
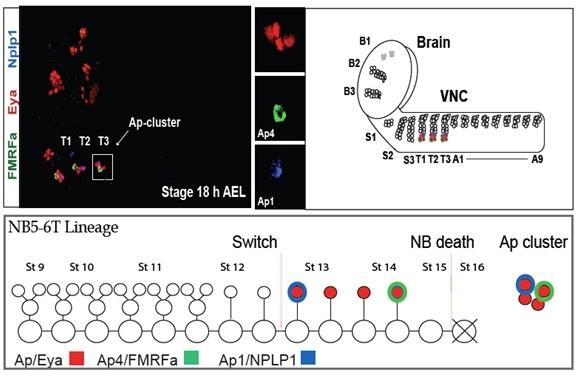
The Ap cluster is placed in all six thoracic hemisegments and is made of four types of neurons AP1-4, (Lundgren et al. 1995), which are specialized from neouroblast 5-6T lineage (Baumgardt et al. 2007). Specification of AP cluster crucially requires some special regulator factors like BMP. Ap cluster generates in a restrictive pattern regulated by transcriptional elements. AP neurons subtypes are distinguishable from each other by differences in their molecular profile (Baumgardt et al. 2009) e.g. FMRFamide neuropeptide is a terminal gene exclusively expressed in the last differentiated neurons within the Ap cluster. FMRFa stereotype expression pattern (Schneider et al. 1991) is under control of the Tv-enhancer (Benveniste & Taghert 1999). In this study Ap4/FMRFa and other terminal and selector genes like Eye absent, Ap/Eya and Ap1/NPLP1 are utilized as proper markers, for the entirely correct specification of AP cluster identification.
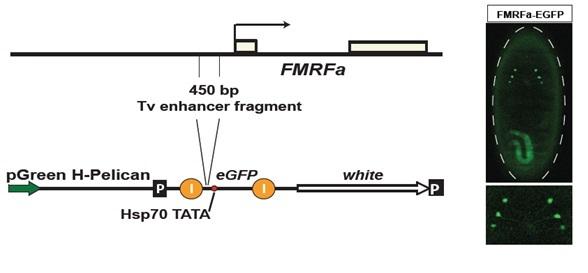
EMS mutagenized and homozygozied for the mutant chromosome had got TV-GFP insertion. Further, they were screened for alternation on FMRFa-GFP expression at the late embryonic stage ( Ulvklo 2009)
Responsible for this page:
Director of undergraduate studies Biology
Last updated:
05/18/12
