Background
Heparan Sulfate
Heparan sulfate (HS) is a glycosaminoglycan and is structurally similar to heparin. Modifications by several enzymes allow for the synthesis of a vast number of different patterns of the HS chain, which are then specifically recognized by certain molecules like growth factors.
HS is attached to a core protein, forming a proteoglyan (heparan sulfate proteoglycans - HSPGs). HSPG’s are found in the extracellular matrix, on the cell surface or in mast cells. Localized on the cell surface, HSPGs serve important functions as mediators in processes like cell differentiation and migration for example. It is also known, that HSPG's promote the interaction between growth factors and their respective receptors. By altering the structure of heparan sulfate, for example by knocking out the biosynthesis enzyme Ndst-1, which mediates the replacement of acetylgroups with sulfates, the net charge of the heparan sulfate chain will be more positive and hence due to less electrostatic forces to each other interactions to growth factors will be decreased.
Histidine-rich glycoprotein
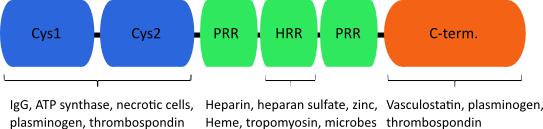
Histidine-rich glycoprotein (HRG) is an abundant plasma protein, which is involved in numerous cellular processes such as the modulation of cell- adhesion, formation of immune complexes, coagulation and angiogenesis. The protein can be structurally divided in three domains, two N-terminal cysteine protease inhibitor like stretches, a histidine/proline rich domain and the C-terminus. A variety of molecules have been shown to interact with HRG, among them divalent cations, plasminogen, DNA, heparin and heparan sulfate.
The implication that HRG may serve an important role in angiogenesis highlights it as a potential therapeutic target in pathological conditions like cancer. It has been demonstrated that HRG-deficient mice show higher tumor volumes at the age of 12 and 15 weeks in the Rip1-Tag2 background.
A possible explanation for a mechanism of action could be competition for binding to HS of growth factors and HRG. Additionally, it was demonstrated that anti-angiogenic capacity is conferred by interference with focal adhesions. Consequently, the ability of endothelial cells to migrate is attenuated and adhesion is decreased. There is evidence that the His/Pro-rich domain can bind to HS. The His/Pro-rich domain can be proteolytically released from the protein and exerts earlier described effects. The minimal active domain was further narrowed down to a 35 amino acid long fragment, HRG330. This peptide is able to confer anti-angiogenic effects in vitro and in vivo, and therefore has a potential as a therapeutic target.
Animal models
The Rip1-Tag2 mouse model
The Rip1-Tag2 mouse is a widely used mouse strain, modelling a system of multistage carcinogenesis. In this model, spontaneous insulinomas are formed in the islands of Langerhans. This is achieved by expression of the large T-antigen from the SV40 virus under the control of the rat insulin promotor. Crossbreeding a Rip1-Tag2 mouse with a floxed Ndst-1 gene with a mouse harbouring the TecCre recombinase, results in selectively knocked out Ndst-1 in the endothelium of these tumor forming mice. Thus endothelial HS will be less sulfated, allowing for studies on how this affects blood vessel formation.
Zebrafish as a model organism for vasculogenesis
The development of the zebrafish (Danio rerio) has been well characterized. Due to easily performed genetical modifications and a short generation time, the zebrafish advanced to a suitable model organism for vertebrate development, drug testing and regenerative medicine for instance.
The transparency of embryos and a complex circulatory system comparable to mammals contributed to promote the zebrafish to a convenient model to study physiological as well as pathological angiogenesis. In addition, embryos express a fairly homologous variant of vascular endothelial growth factor, in comparison to the mammalian VEGF. Visualization of the vasculature is easily achievable, for example through staining for endogenous alkaline phosphatase activity in early developmental stages. By injection into the common cardinal vein (CCV), the effect of pro- and antiangiogenic factors can be observed by investigating vessel alterations of the sub-intestinal basket.
Responsible for this page:
Director of undergraduate studies Biology
Last updated:
05/23/13
