Methods
Dissectioning of mice and measuring of tumor volumes
Mice were anesthetized with 2% intraperitoneally injected avertin. Heart perfusion was performed with 10 ml 1x PBS followed by 10ml of 2% PFA in PBS. The pancreas was then dissected from the abdominal cavity. Exocrine pancreas was removed and tumors and angiogenic islets were counted and measured under a stereo dissection microscope. Islets were defined by a diameter of < 1mm. Tumor volumes were calculated according to the formula ((π/6) x width^2 x length). A two-tailed Mann-Whitney test was conducted for statistical analysis.
Gel-shift assay
To investigate the ability of HRG-derived peptides to bind to heparin, a gel-shift assay was conducted. Samples containing heparin, HRG-derived peptides, 1x TBS were made up to a final volume of 10 μl with dH2O and incubated for one hour at room temperature. 10x loading buffer was added to a final concentration of 1x. gels. The samples were separated on 6% stacking and 20% separating gels and were run for 75 minutes at 200V in 1x TBE. Next, staining for one hour with 0.5% alcian blue was performed. To decrease background, gels were washed for one hour in destaining solution, followed by three washing steps in dH20, each 15 minutes. Following this, the gel was immersed in silver stain for one hour, then washed three times for 20 minutes in dH20. For development of the bands, the gel was constantly shaken in development solution until the desired degree of intensity of the bands was reached. The reaction was stopped by the addition of 0.5% acetic acid to the development solution.
Zebrafish sub-intestinal basket assay
AB strain (wild type) embryos were collected and incubated at 28°C in embryo water containing 60 μg/ml instant ocean sea salts and 0.05% methylene blue until 48 hours post fertilization (hpf). Under a stereo microscope, fish were injected into the common cardinal vein (CCV) with either HRG330 or phenolred. Embryos were then incubated in embryo water for one day (until 72 hpf) and then fixed and stained.
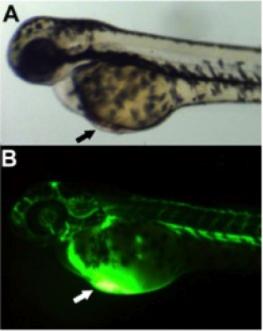
Staining for endogenous alkaline phosphatase activity
Embryos were taken out of the embryo water at 72 hpf, rinsed briefly in PBS and fixed for one hour at room temperature in 1x PBS + 4% PFA + 1% Triton X. Following, fixative was removed and the embryos were washed for 5x 10 mins in PBS containing BSA and Triton-X. Samples were then incubated in freshly prepared NTMT twice. Embryos were transferred to fresh NTMT and then stained for alkaline phosphate activity by the addition of nitrotetrazolium blue chloride (NBT) and X-Phosphate. Incubation was for 15 minutes wrapped in aluminium foil. To wash away residual NBT and X-phosphate, samples were suspended in 1x PBS + 1% BSA + 0.5% Triton-X. In order to remove pigments from melanocytes, embryos were suspended in bleaching solution. After 15 mins of bleaching, embryos were briefly rinsed in PBS and stored at 4°C.
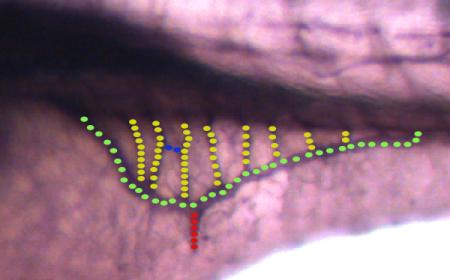
Quantification and measurement of vessels
Fish embryos were mounted in 3% methylcellulose. Images of the whole length of the fish were taken at 10X magnification, the sub-intestinal baskets were captured at 100X magnification with a stereo microscope. Analysis and measurements were carried out with the AxioVisionLE software, provided by Zeiss.
Responsible for this page:
Director of undergraduate studies Biology
Last updated:
06/03/13
